Investing in the research of drug molecules has and continues to be one which has a high degree of risk. Despite a growing pharmaceutical market, and increasing numbers of trials, the reticence of lenders and investors to fund Phase 1 and Phase 2 trials has held back innovation and led to a model which is extremely difficult to navigate.
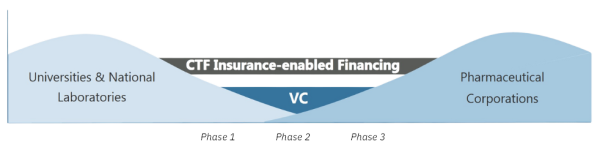
Through a combination of traditional underwriting expertise, cutting edge technology and some of the insurance market’s leading clinical trials practitioners, MCI seeks to mitigate this risk, thereby opening up the financing world more effectively and giving innovators the chance to retain greater control of their business.
What We Offer:
- Insurance covering the costs incurred by the biotech company in a clinical trial, which has failed to meet its primary protocol endpoints
- Costs covered include but are not limited to: CRO costs, protocol design fees and hospital/medical practitioner costs associated with design, implementation, monitoring and review of trial results
- Access to lenders and financiers of clinical trials, who consider the insurance valid security*
*MCI can refer those interested to the necessary regulated entities but cannot advise on, or negotiate financial instruments (other than the CTF insurance itself)
What the insurance enables
- Reduced risk for Investors and lenders, allowing potentially:
- Retention of more equity for the drug asset Innovator; and
- Greater control of your business, rather than being traded as another drug in a large portfolio
- Quicker, streamlined financing process improving chances of being first-to-trial (<4 weeks for an indication)
- Greater choice of equity investors, through opening up those with lower appetites for risk
- Greater chance of getting funding. We estimate between 30% and 50% of risks considered will be offered terms (as opposed to <10% in typical CT funding scenarios)
What we target
- Phase 1 and Phase 2 trials
- Trial budgets from $3M – $20M
- Drug asset type – small molecule, monoclonal antibodies, some biologics** Trials with an estimated duration of less than 3 years
- Companies that have already engaged with a CRO
- Trials that have their Clinical Trial Protocol already written and approved by the relevant regulator
- Companies domiciled in USA, UK and Canada
**Unable to support Gene Therapies at this time